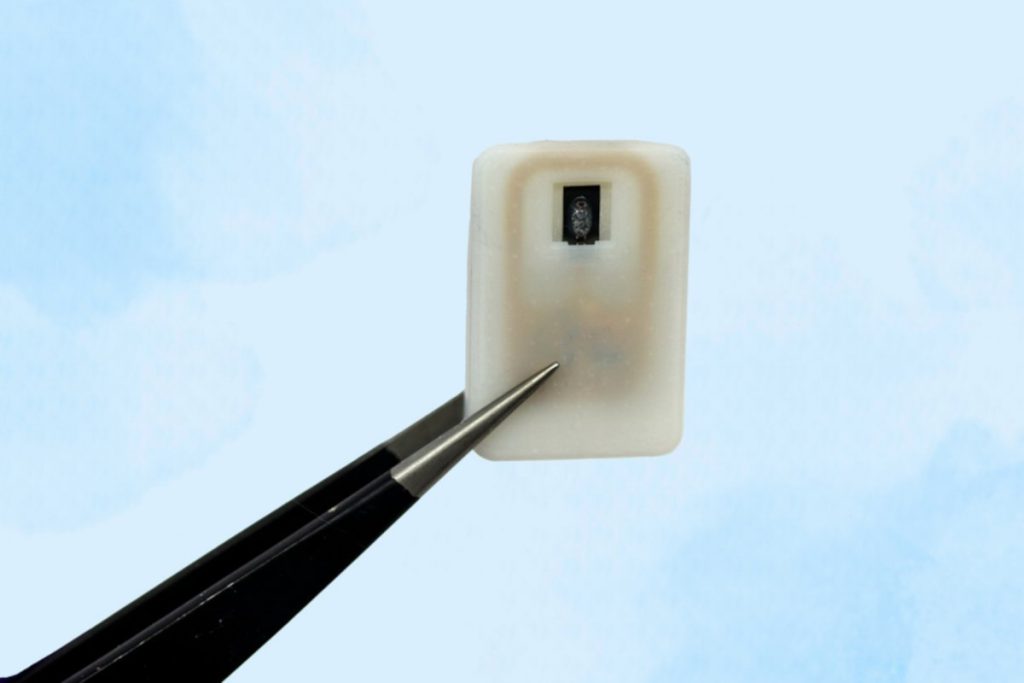
Scientists at the Massachusetts Institute of Technology (MIT) have developed an emergency implant for people suffering from type 1 diabetes. The implant can deliver a dose of glucagon in emergencies to stabilize blood sugar levels and protect against life-threatening hypoglycaemia.
People with type 1 diabetes are at constant risk of their glucose levels dropping too low. In the worst-case scenario, this can be life-threatening and lead to death. An injection of glucagon, a peptide hormone that stimulates alpha cells in the liver to break down glycogen and at the same time causes stored glucose to be released into the bloodstream, can protect against this.
Many people with diabetes notice for themselves when something is wrong and can then help themselves by taking an emergency medication. However, children often do not notice this. They usually do not recognize the first signs. All diabetics are also at risk during sleep.
This is where the MIT implant comes into play. This is a small device that is implanted under the patient’s skin and can release glucagon as soon as the blood sugar level drops too low and the patient is unable to administer the glucagon quickly. This includes automatic administration during sleep.
The small device consists of a 3D-printed drug container made of a polymer that is sealed with a metal component made of nickel-titanium. The researchers write in the study “Emergency delivery of particulate drugs by active ejection using in vivo wireless devices,” which has been published in Nature Biomedical Engineering. The alloy rolls up at a temperature of 40 °C. The necessary heating of the seal is activated remotely via an external wireless connection. A small electric current brings the metal seal up to temperature and opens it. The glucagol contained in the medication chamber is then released and triggers the necessary processes in the diabetic’s body.
Durable glucagon in powder form
Instead of using liquid glucagon, the researchers use powdered glucagon. The advantage: the powder version remains stable for longer. The researchers expect the glucagon to last for a year. Thereafter, it must be replaced. However, it may also last longer. According to the MIT scientists, more detailed investigations are still required.
The device can be triggered manually or automatically via external sensors that diabetes patients often use to monitor their blood glucose levels.
The research team tested the emergency implant on diabetic mice. After activation, their blood glucose levels dropped within ten minutes, stabilized, and remained within the normal range. The device worked reliably even when scarring had occurred. The researchers also tested the administration of other drugs, such as adrenaline in powder form. The heart rate was also increased within ten minutes of the implant being triggered.
The researchers removed the implant after four weeks. They are now working on increasing the lifespan of the device and thus its retention in the body. The scientists estimate that the device can remain in the body for between one and several years. However, they do not yet know the exact details. They are working on determining the optimum lifespan. In any case, the implant will have to be replaced at some point.
However, it will be some time before the emergency implant can be used in medicine. The MIT researchers assume that it can be tested on humans in around three years’ time.
(olb)
Don’t miss any news – follow us on
Facebook,
LinkedIn or
Mastodon.
This article was originally published in
German.
It was translated with technical assistance and editorially reviewed before publication.
Dieser Link ist leider nicht mehr gültig.
Links zu verschenkten Artikeln werden ungültig,
wenn diese älter als 7 Tage sind oder zu oft aufgerufen wurden.
Sie benötigen ein heise+ Paket, um diesen Artikel zu lesen. Jetzt eine Woche unverbindlich testen – ohne Verpflichtung!