Electric vehicles are thriving globally, with sales and market penetration continuing to rise. This also means that a mountain of electronic waste will accumulate in the future. Despite many efforts to improve battery recycling, many electric vehicle batteries still face the fate of being landfilled.
Batteries consist of three main components: a positively charged cathode, a negatively charged anode, and an electrolyte that transports lithium ions between them. The electrolytes in most lithium-ion batteries are highly flammable and degrade into toxic byproducts over time, requiring special handling. As a result, recycling batteries generally involves harsh chemicals, high temperatures, and complex processes.
Recently, the team led by MIT’s Yet-Ming Chiang and Julia Ortony aims to change this situation with a new type of self-assembling battery materialthat can rapidly decompose when immersed in a simple organic liquid. In a paper published in the journal Nature Chemistry, the researchers demonstrated that this material can serve as an electrolyte for solid-state batteries in normal operation and can revert to its original molecular state within minutes.
This approach provides an alternative to crushing batteries into hard-to-recycle mixtures. Since the electrolyte acts as the connective layer in the battery, when the new material returns to its original molecular form, the entire battery self-decomposes, thus accelerating the recycling process.
“So far, in the battery industry, we have focused on high-performance materials and designs, then tried to figure out how to recycle those structurally complex and difficult-to-recycle batteries,” said Yukio Cho, the paper’s first author and a Ph.D. student graduating in 2023. “Our approach starts with easily recyclable materials and then explores how to make them compatible with batteries. Designing recyclable batteries from the outset is a novel method.”

Designing Recyclable Electrolytes
In the Harry Potter series, there is a scene where Professor Dumbledore can clean up a dilapidated house with just a wave of his wrist and a spell. This image left a deep impression on Yukio Cho during his childhood.
After attending Julia Ortony’s talk on designing molecules to assemble into complex structures and then revert to their original form, he began to ponder whether battery recycling could also be magically simplified.
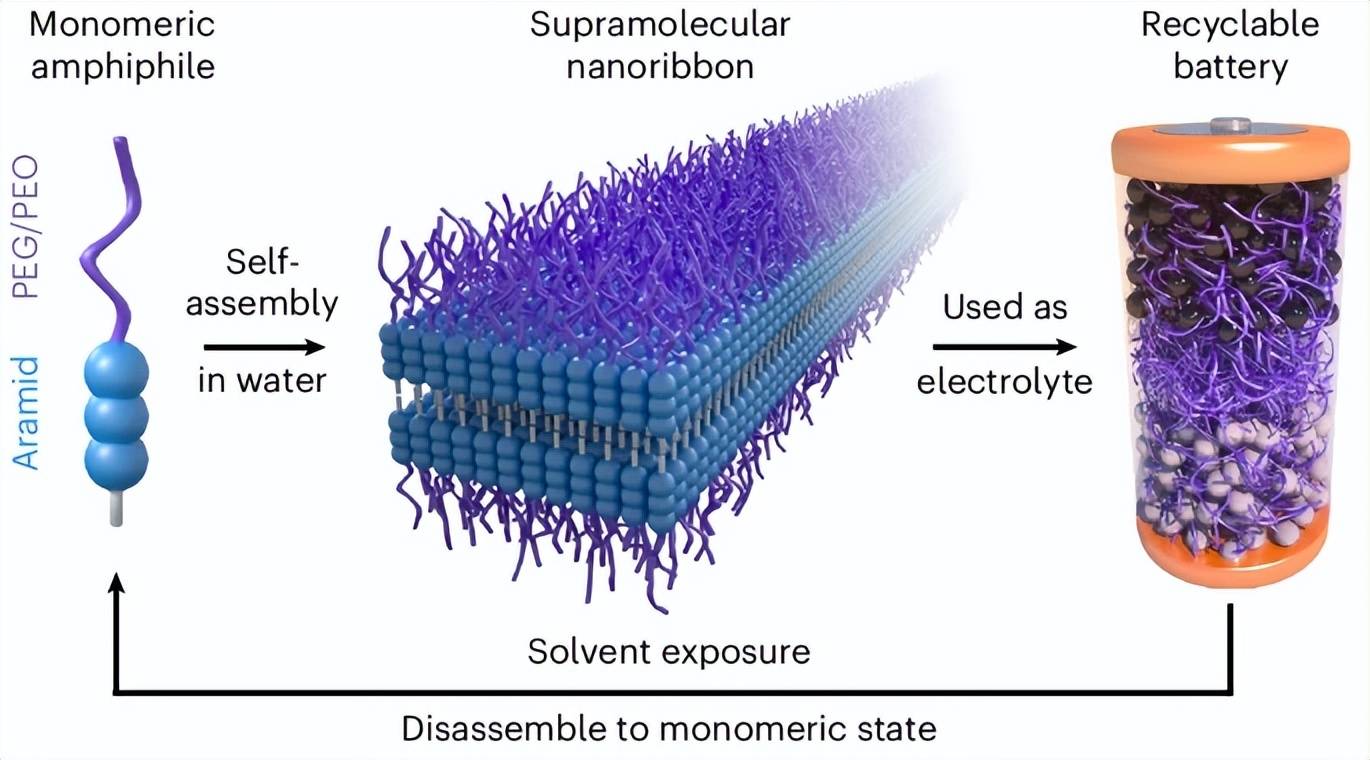
To streamline the recycling process, the researchers decided to create a more sustainable electrolyte. To this end, they studied a class of molecules that self-assemble in water, known as aromatic amphiphiles (AAs), whose chemical structure and stability are similar to Kevlar. The researchers modified AAs to include polyethylene glycol (PEG) segments to facilitate lithium ion transport. When exposed to water, the resulting molecules self-assemble into mechanically stable nanoribbons with ion-conductive PEG surfaces.
“This material consists of two parts,” Cho explained. “The first part is this flexible chain that provides a carrier for lithium ions to hop. The second part is a strong organic material component used in Kevlar fibers, which is a bulletproof material. These two parts stabilize the entire structure.”
When added to water, the nanoribbons self-assemble into millions of nanoribbons. These assembled nanoribbons form a gel-like material that can be pressed into a flexible solid electrolyte. During battery operation, this material supports the flow of lithium ions between the battery electrodes. However, when placed in an organic solvent, the nanoribbon structure rapidly disassembles, allowing for easy battery disassembly.

Validating the New Method
The team tested the strength and toughness of this material and found that it could withstand the pressures associated with manufacturing and operating batteries. They also built a functional solid-state battery using lithium iron phosphate as the cathode and lithium titanate as the anode—both materials already used in commercial batteries. They found that the PEG-based nanoribbons could successfully transport lithium ions in the electrolyte.
However, tests indicated that due to polarization effects, the rate at which ions entered the electrodes was slower than ideal. This effect limits the battery’s performance during fast charging or discharging.
“Lithium ions can indeed move along the nanofibers, but transferring lithium ions from the nanofibers to the metal oxide seems to be the slowest part of the entire process,” Cho said. However, as a proof of concept, the researchers were satisfied with the performance their battery could achieve.
In his view, there is significant room for optimizing the performance of this material through further experiments.
Currently, the researchers are exploring ways to integrate this type of material into existing battery designs while also applying these ideas to new battery chemistries.
“Convincing existing suppliers to do things in a very different way is very challenging. But for new battery materials that may emerge in the next five to ten years, it may be easier to integrate them into new designs from the start.”
Cho also believes that this approach could help recycle lithium back to the U.S. by reusing materials from existing batteries. “People are starting to realize how important this is. If we can begin large-scale recycling of lithium-ion batteries from battery waste, it would have the same effect as opening lithium mines in the U.S. Moreover, each battery requires a certain amount of lithium, so inferring from the growth of electric vehicles, we need to reuse this material to avoid a significant spike in lithium prices.”
Original link:
1. https://news.mit.edu/2025/new-self-assembling-material-could-be-key-recyclable-ev-batteries-0828返回搜狐,查看更多

